
NPHarvest Nutrient Catcher System
The NPHarvest process allows for the recovery of nitrogen and phosphorus from wastewater, transforming wastewater from an environmental burden into a source of reusable resources.
Description
Turning waste into opportunity – all in one flow
Let’s take a closer look at the process
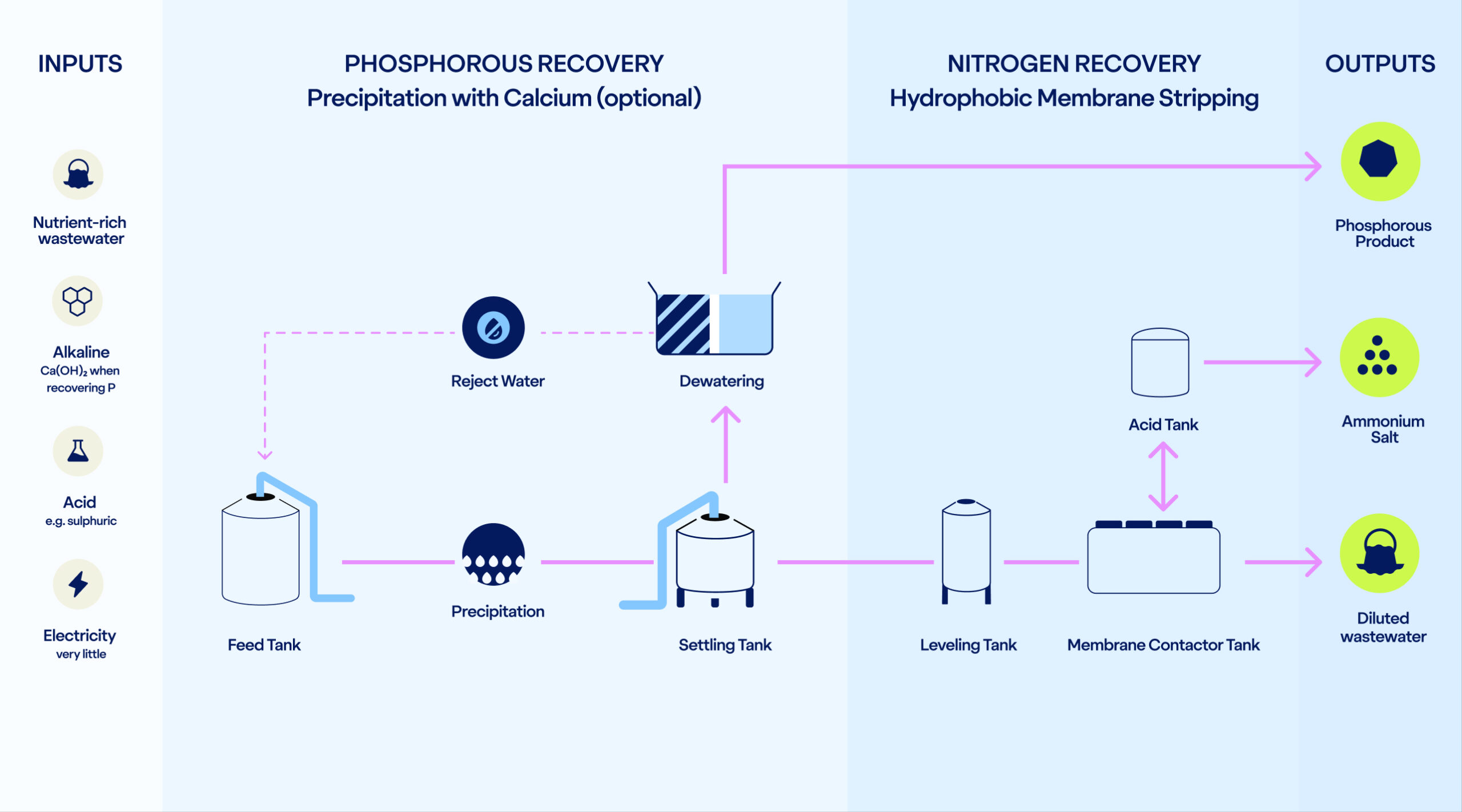
Inputs
Setting the stage for nutrient recovery
The process requires three core inputs: an alkaline, an acid, and the nutrient-rich liquid waste stream. The flexibility in alkaline and acid choice allows the process to adapt to local conditions.
When P recovery is included, the alkaline used is calcium hydroxide. With N recovery alone, any alkaline such as sodium hydroxide can be used.
An acid is needed to bind the ammonia into an ammonium salt. The most typical acid to use is sulphuric acid, creating ammonium sulphate. However, several acids can be used depending on the desired end-product.
Last (but not least), the process takes in the concentrated wastewater. Economic feasibility starts from a +500g/m³ nitrogen concentration.
In addition, electricity is required to pump the water (under 1 bar pressure) through the system, and LKD can be used to enhance the phosphorus precipitation.
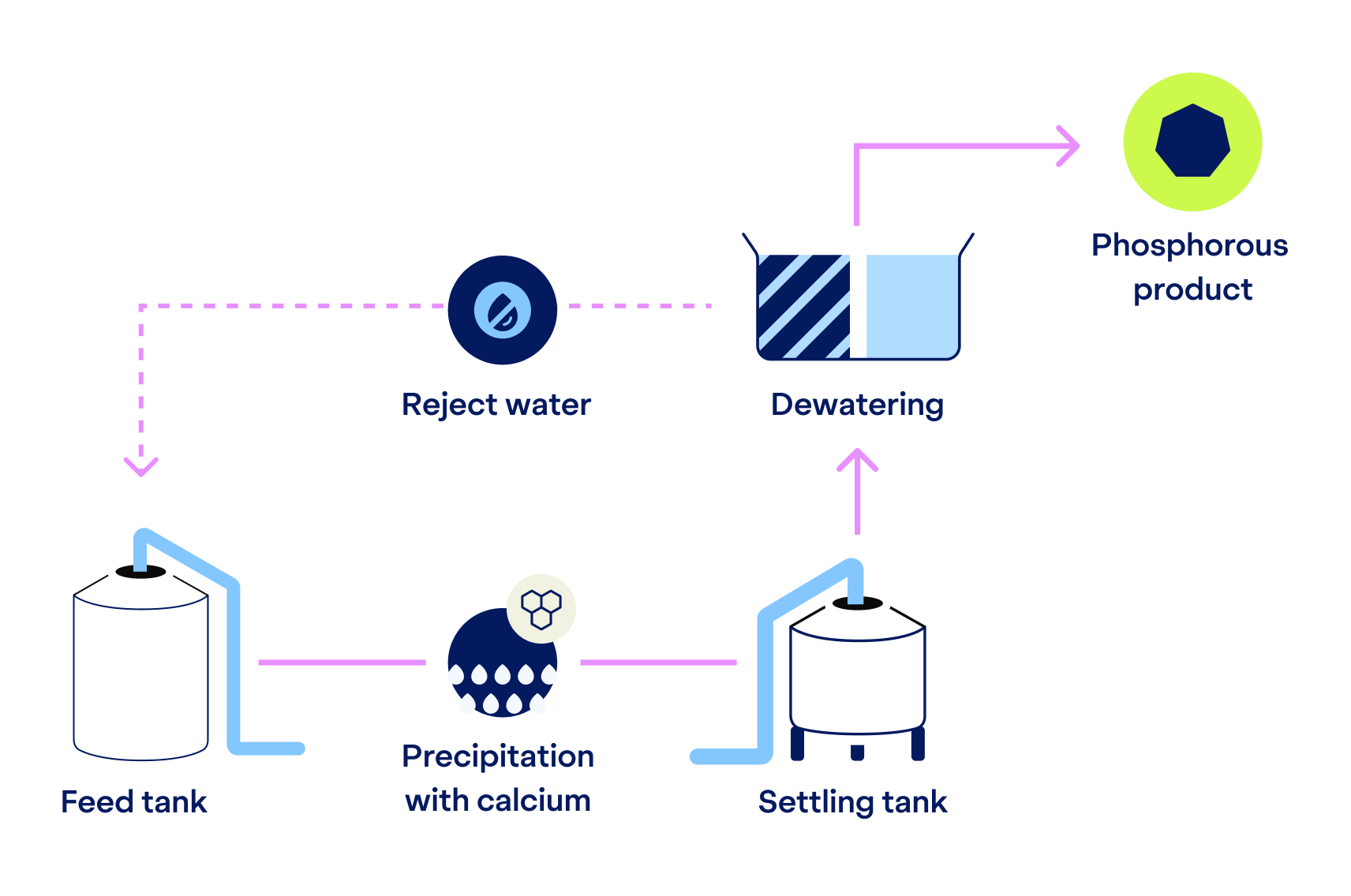
Phosphorus Recovery
Creating a local phosphorus source
If phosphorus recovery is desired, we dose calcium hydroxide to the wastewater to enable precipitation with calcium. Simultaneously, we get a double benefit: the pH increases to support the following nitrogen recovery step.
The phosphorus is then separated as a solid via settling and dewatering. The end-product is amorphous calcium phosphate that can be reused – for example, as a component of fertilizers.
When phosphorous recovery is not required, only the alkaline dosing of this step occurs, making the process flexible depending on the client’s needs.
There is no significant pre-treatment needed. The process can handle TSS up to 3% for both phosphorus and nitrogen recovery – unlike many other recovery solutions.
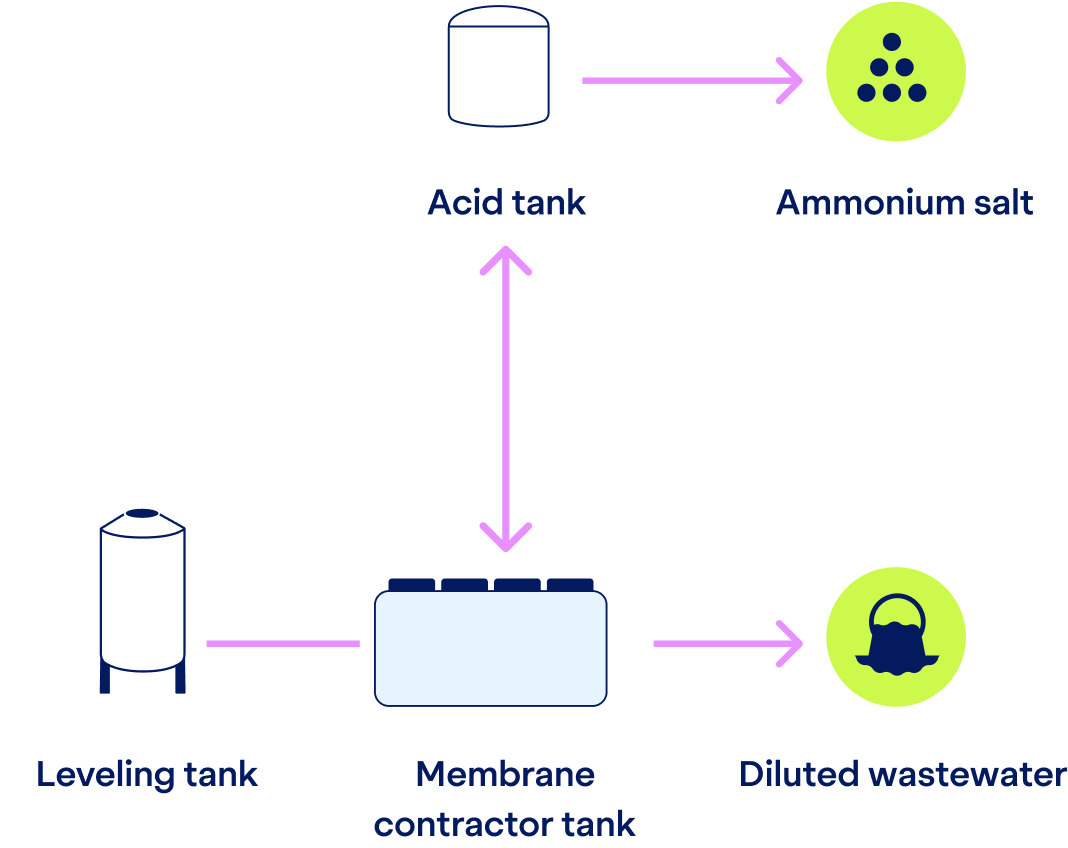
Nitrogen Recovery
Capturing valuable nitrogen
After phosphorus is handled, the remaining wastewater undergoes nitrogen recovery.
Through a stripping process, ammonia is extracted from the water. The previous pH increase makes ammonia volatile, and a concentration difference makes the gaseous ammonia pass through our hydrophobic membranes.
On the other side of the membrane, we introduce an acid, such as sulfuric acid, depending on local availability and desired end-product. The ammonia reacts with the acid, forming a valuable ammonium salt solution, which can be repurposed for various agricultural and industrial uses.
Don’t mistake our stripping membranes for filtering membranes. Because the ammonia is stripped (not filtered) and there’s no pressure increase, fouling or clogging aren’t an issue — unlike with many filtering membranes.
Outputs
Water and nutrients are cleanly separated
At the end of the process, we achieve three outputs:
- Phosphorus product
- Ammonium salt
- Diluted wastewater
This integrated approach transforms wastewater from an environmental burden into a source of reusable resources.
Our process only tackles nitrogen and phosphorus. The wastewater will still need to be treated as it normally would for other pollutants.
Benefits
Low Operational Costs
Our technology requires only increasing pH – no heating, aeration, pressure, or additional chemicals.
High-quality end-product
We recover nitrogen as a pure ammonia salt and phosphorus as amorphous calcium phosphate.
Easy and reliable
Our process is physicochemical and operated only by dosing an alkaline and an acid. It does not clog with TSS up to 3%.


